Given the following list of Ka values, determine which weak acid solution would have the lowest pH value at equilibrium assuming all acids have the same initial concentration. 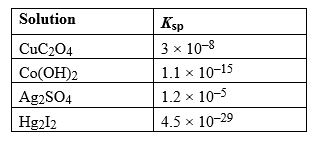
Definitions:
Double-Declining-Balance
A method of accelerated depreciation which doubles the normal depreciation rate.
Scrap Value
An estimated calculation of what an asset will be worth at the time it is sold, after it is no longer useful.
Sum-Of-The-Years-Digits
An accelerated method of depreciation which totals the digits of an asset's useful life and allocates the cost based on a fraction of those digits.
Scrap Value
The calculated resale value of an asset at the termination of its functional life.
Q5: Which state of matter has particles that
Q8: Write and balance the equation for the
Q11: _ are pure substances.<br>A) Homogeneous mixtures and
Q14: Assign the correct class name to each
Q18: Virtually all scientists agree that global warming
Q30: When a third party assists both sides
Q34: Lavoisier performed many of the same experiments
Q39: Which of the following is true of
Q70: When 28 g of nitrogen and 6
Q141: The words science and technology are often