Given the following list of Ka values, determine which weak acid solution would have the highest pH value at equilibrium assuming all acids have the same initial concentration. 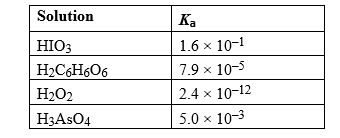
Definitions:
True Score
The actual score that accurately reflects the tested attribute or characteristic without error.
Reliable Measure
A consistent and dependable method of measurement that yields the same results under similar conditions.
Measurement Error
The difference between a measured value and the true value that can result from various inaccuracies or biases.
Reliable Measure
A method or instrument that consistently produces stable and accurate results over time.
Q1: What is the formula for the alkene
Q4: The GATT principle that called for mutual
Q5: There is no difference between the hazard
Q9: Carbon-14 dating can be used to determine
Q18: Write the condensed structural formula for hexylpentylamine.
Q26: Because human rights have been enumerated in
Q36: Mercantilists differ from liberalists in that<br>A) mercantilists
Q40: What are the risks involved in using
Q53: Most of the fiscal crises in the
Q174: The mass of a nucleon is approximately