The chemical acidity of a solution is measured in units of pH: 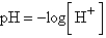 , where
, where 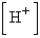 is the hydrogen ion concentration in the solution. If a sample of rain has a pH of 3.3, how many times higher is its
is the hydrogen ion concentration in the solution. If a sample of rain has a pH of 3.3, how many times higher is its 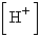 than pure water's, which has a pH of 7?
than pure water's, which has a pH of 7?
Definitions:
Loop of Henle
A segment of the nephron in the kidney crucial for concentrating urine and conserving water.
Afferent Arteriole
The small artery that carries blood toward the capillaries in the glomerulus of the kidney.
Glomerulus
A network of capillaries located in the beginning of a nephron in the kidney where blood filtration takes place.
Vasa Recta
Specialized capillary that extends from the cortex of the kidney into the medulla and then back to the cortex.
Q8: Find the center and vertices of the
Q16: Find the vertex of the parabolic graph
Q33: Find the exact value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8632/.jpg"
Q48: Which of the following is a zero
Q62: Solve the following equation. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8632/.jpg" alt="Solve
Q80: The monthly sales S (in hundreds of
Q135: Using the figure below, if <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8632/.jpg"
Q142: Find the center and vertices of the
Q143: Solve <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8632/.jpg" alt="Solve by
Q146: Find the vector v that has a