The radioactive bismuth isotope 210 Bi has a half-life of 5 days. If there is 100 milligrams of 210 Bi present at t = 0, then the amount f(t) remaining after t days is given by: 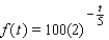 How much 210 Bi remains after 5 days?
How much 210 Bi remains after 5 days?
Definitions:
Initial Outlay
The initial investment amount required to start a project or purchase an asset.
Future Cash Inflows
Expected incoming funds or earnings generated from investments, operations, or financial activities in the future.
Discount Rate
The discount rate that's used to estimate the present value of future cash flows within discounted cash flow analysis.
Net Present Value
Net Present Value (NPV) is a financial metric used to evaluate the profitability of an investment, calculated by summing the present values of incoming and outgoing cash flows over a period of time.
Q16: Find an equation of a rational function
Q22: An aspirin tablet is in the shape
Q24: Determine the range of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8634/.jpg" alt="Determine
Q54: As <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8634/.jpg" alt="As for
Q90: Solve the inequality. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8634/.jpg" alt="Solve the
Q94: Change to exponential form. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8634/.jpg" alt="Change
Q105: Use the graph of f to find
Q123: A triangular field has sides of lengths
Q141: Use common logarithms to solve for x
Q146: Points on the terminal sides of angles