The amount of heat needed to change the temperature of an object can be calculated using the following equation: 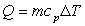 where
where 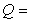 amount of heat in Joules (J)
amount of heat in Joules (J) 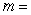 mass of object in kilograms (kg)
mass of object in kilograms (kg) 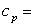 specific heat of object
specific heat of object 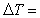 change in temperature in °C
change in temperature in °C
What is the appropriate unit for 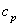 if the preceding equation is to be homogeneous in units?
if the preceding equation is to be homogeneous in units?
Definitions:
Q3: The largest phylum in the animal kingdom
Q7: Materials engineers research, develop, and test new
Q7: Which of the following statements accurately represents
Q8: The young of sugar gliders are called
Q10: What species of mammals should be considered
Q17: Pressure is a fundamental dimension.
Q19: A force of 20 N causes a
Q21: A conflict of interest is<br>A)a general disagreement
Q23: The computer-generated solid models save time and
Q29: List at least four of the eight