In a gas mixture, the partial pressures are:  76.0 torr, He 230 mmHg , and
76.0 torr, He 230 mmHg , and 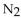 0.640 atm. The total pressure in mmHg is
0.640 atm. The total pressure in mmHg is
Definitions:
Cohorts
Groups of individuals sharing a common characteristic or experience within a defined period, often used in studies to examine the effects over time.
Null Hypotheses
Hypotheses that state there is no significant difference or relationship between specified populations, any observed difference being due to sampling or experimental error.
Main Effect
The direct influence of an independent variable on a dependent variable in an experiment, ignoring the effects of all other independent variables.
Factorial ANOVA
A statistical method designed to evaluate the impact of two or more variables acting independently at various levels on an outcome variable.
Q6: What is the total number of ATP
Q10: Which of the following represents an opportunity
Q13: To synthesize a <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="To synthesize
Q13: Gus is keeping a reflection journal during
Q16: A cyclopropane-oxygen mixture is used as an
Q23: What element has the electron configuration <img
Q36: What is the coefficient for oxygen in
Q50: Myristic acid, a <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Myristic acid,
Q54: What is the correct formula for the
Q59: 0.50 mole of KCl, a strong electrolyte,