For the following reaction, the equilibrium constant 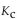 is 2.0 at a certain temperature. Bromine can be liquefied easily and removed from the reaction vessel as it is formed. If this is done, how will it affect the equilibrium reac
is 2.0 at a certain temperature. Bromine can be liquefied easily and removed from the reaction vessel as it is formed. If this is done, how will it affect the equilibrium reac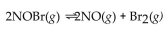
Definitions:
Capital Expenditures
The costs of acquiring fixed assets, adding to a fixed asset, improving a fixed asset, or extending a fixed asset’s useful life.
Fixed Asset
Long-term tangible property that a company owns and uses in its operations to generate income, such as buildings, machinery, and equipment, which are not expected to be consumed or converted into cash within a year.
Property, Plant, Equipment
Long-term assets held for business use and not intended for resale, such as buildings, machinery, and vehicles, subject to depreciation.
Depreciated
A decrease in the value of an asset over time due to wear and tear or obsolescence.
Q14: In which of the following are the
Q20: Which of the following statements is the
Q23: A molecule containing a carbon atom bonded
Q25: What is the name of this compound?
Q27: What type of alcohol undergoes oxidation to
Q30: In the reaction of carbon dioxide with
Q46: What is the product of the reaction
Q47: When naming an alkene, the parent chain
Q55: The marginal rate of transformation is<br>A)is equal
Q56: A permanent increase in income leads to<br>A)a