The equilibrium constant for the reaction for the decomposition of 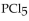 to chlorine and
to chlorine and 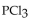 at a certain temperature is 0.042 .
at a certain temperature is 0.042 .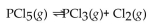
If the equilibrium concentrations are 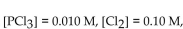 what is the value of
what is the value of 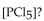
Definitions:
Enthusiasm
Intense and eager enjoyment, interest, or approval.
Yerkes-Dodson Law
A theory that suggests there is an optimal level of arousal for performance, and that too little or too much arousal can adversely affect task performance.
Mental Arousal
The psychological state of being alert and mentally engaged, often in response to stimulation or challenge.
Physical Arousals
Pertains to bodily reactions or physiological responses to stimuli.
Q2: The steroid hormone present in birth control
Q3: In the endogenous growth models of Lucas
Q6: The Ricardian Equivalence Theorem implies that a
Q8: Which of the following is a buffer
Q12: When consumption and leisure are both normal
Q13: If the consumer is a lender
Q16: The Malthusian model has the property that<br>A)increased
Q44: A permanent decrease in taxes leads to<br>A)no
Q57: A hydrocarbon with at least one double
Q61: In the three-dimensional structure of methane, <img