In the following gas phase reaction, Kc is much less than 1. At equilibrium, which of the following statements is true? 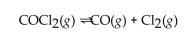
Definitions:
Cash Flows
The total amount of money being transferred into and out of a business, affecting its liquidity.
Net Working Capital
The difference between a company's current assets and current liabilities, indicating its short-term financial health and operational efficiency.
Present Value
The calculation of the current value of a sum of money or stream of cash flows that is to be received in the future, discounted at a specific rate.
Dollar Outlays
The total amount of money spent or invested in a particular project, purchase, or endeavor, often used in budgeting and financial planning.
Q4: Perfect substitutes will have<br>A)reverse L-shaped indifference curves.<br>B)curved
Q6: Which of the following is NOT different
Q7: In equilibrium, an increase in Employment Insurance
Q8: Physiologically active nitrogen-containing compounds produced by plants
Q33: "More is always preferred to less" refers
Q36: What is the IUPAC name of this
Q43: In the figure above, the monosaccharide unit
Q55: The IUPAC name for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q58: The name given to an aqueous solution
Q59: Suppose Ford could triple production of Mustangs