For the following reaction, the equilibrium constant 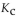 is 0.60 at a certain temperature. If the concentration of NO(g) and NOBr(g) are both 0.50 M ,at equilibrium, what is the concentration of
is 0.60 at a certain temperature. If the concentration of NO(g) and NOBr(g) are both 0.50 M ,at equilibrium, what is the concentration of 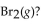
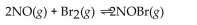
Definitions:
Inventory
The total amount of goods and materials held by a business for the purpose of resale or production.
Fixed Costs
Expenses that remain constant regardless of business activity levels, including long-term leases, salaries, and insurance premiums.
Variable Cost
A cost that varies with the level of output or production volume.
Volume of Activity
The level of production or sales volume at which a business operates during a specific period.
Q5: What is the name of the medical
Q9: Which of the following is the correctly
Q11: For the equation 4x + 2 =
Q11: An increase in total factor productivity involves<br>A)more
Q27: There is more traffic between 8:00 and
Q34: What is the common name of this
Q37: Written in scientific notation, 8300 is <br>A)
Q39: The real wage is<br>A)acyclical.<br>B)higher than real GDP.<br>C)countercyclical.<br>D)procyclical.<br>E)always
Q48: Which solution has the lowest pH?<br>A)a buffer
Q53: What is the IUPAC name of this