For the following reaction, the equilibrium constant 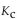 is 2.0 at a certain temperature. Bromine can be liquefied easily and removed from the reaction vessel as it is formed. If this is done, how will it affect the equilibrium reac
is 2.0 at a certain temperature. Bromine can be liquefied easily and removed from the reaction vessel as it is formed. If this is done, how will it affect the equilibrium reac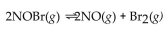
Definitions:
Restriction
A limitation or condition placed on an action, activity, or process.
Promotional Allowances
Financial incentives or discounts offered by suppliers to their distributors or retailers to encourage the promotion of specific products.
Sale Of Securities
The act of selling stocks, bonds, or other financial instruments, typically in the financial markets.
Q11: The Ricardian equivalence theorem implies that<br>A)the timing
Q14: The structure is that of _ acid.<br><img
Q15: Employment is<br>A)more variable than real GDP.<br>B)about as
Q18: In the United States, the skill premium
Q20: Express using scientific notation: 0.000 860
Q27: Choose the polyunsaturated triacylglycerol from the compounds
Q48: Two key properties of indifference curves are
Q52: Changes in government spending are NOT likely
Q61: In the three-dimensional structure of methane, <img
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8494/.jpg" alt=" A)methanechlorine. B)chloromethane. C)methane