Multiple Choice
In the subshell of the Li2+ ion with orbital quantum number 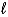 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number 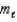 are:
are:
Definitions:
Related Questions
Q3: The wavelength of a 45-kg teenager moving
Q4: Which of the following statements BEST describes
Q4: As an athlete performs a powerful uphill
Q10: A person has 85° of passive right
Q11: Assume a diatomic molecule can be considered
Q12: The switch in the figure is closed
Q13: All particles can be classified into:<br>A)leptons and
Q23: A current may be induced in a
Q25: A ruby laser beam ( <span
Q26: What are the arthrokinematics of the glenohumeral