The number of electrons in the n = 4, = 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, =2 subshell in barium (Z = 56) . 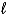
Definitions:
Molecular Formula
A representation of the number and type of atoms in a molecule, without indicating how they are bonded together.
Molecule
The smallest unit of a chemical compound that can exist; composed of two or more atoms bonded together.
Conjugate Acid
A species formed by the reception of a proton by a base, in other words, it is the base with a hydrogen ion added to it.
Methanol
A simple alcohol with the formula CH3OH, known for being a volatile, colorless, and flammable liquid.
Q6: What are the arthrokinematics of the ulnar
Q7: The expectations value of a function f(x)
Q7: A person stands only on the left
Q9: Looking at the image, which muscles are
Q10: Solid argon has a density of 1650
Q11: Which of the following landmarks is used
Q16: The graph below shows the value
Q17: A long solenoid has a radius of
Q30: The laws of refraction and reflection
Q34: A baseball (1 kg) has an