The radial portion of the de Broglie wavefunction for an electron in the ground state of the hydrogen atom is 1s(r) = 1/() 1/2 exp(-r/a0) where a0 is the Bohr radius.The probability of finding the electron is: 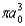
Definitions:
Independent Variables
Independent variables are factors that are manipulated or changed in an experiment to observe their effect on dependent variables.
Optimization Analysis
The process of making something as effective or functional as possible, often through the use of mathematical models to determine the best solution.
CRM Indicators
Metrics or signals used in Customer Relationship Management to track performance, customer satisfaction, loyalty, and profitability.
Regression Logic
Regression logic involves using statistical techniques to model and analyze the relationship between a dependent variable and one or more independent variables.
Q5: The force constant of HCl is 480
Q5: What should be the height of a
Q7: Which muscle action term BEST describes the
Q7: The expectations value of a function f(x)
Q9: A bolt of lightning strikes the
Q23: When light scatters from a rough surface,
Q27: What is the quantum number n of
Q31: A telescope is constructed with two lenses
Q34: Which of the following, in which n
Q45: A plane convex lens is made of