The number of electrons in the n = 4, = 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, =2 subshell in barium (Z = 56) . 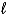
Definitions:
Extraordinary Items
Events and transactions that are distinguished by their unusual nature and by the infrequency of their occurrence, no longer classified separately in financial statements as of recent accounting standards.
Financial Statements
Reports that provide an overview of a company's financial condition, including balance sheet, income statement, and cash flow statement.
Extraordinary Gains
Unusual and infrequent gains that are reported separately from regular income because they result from events that are not part of the company's usual business activities.
Footnote Disclosure
Additional information provided at the bottom of financial statements, offering more detail on specific figures or accounting policies.
Q1: Suppose an object is placed 6.0 cm
Q7: A small airplane with a wing
Q8: What is the typical range of motion
Q9: What arthrokinematics occur during mandibular depression?<br>A)Condyles roll
Q11: An AC generator consists of 6 turns
Q13: A narrow slit is illuminated with
Q21: The intensity of radiation reaching the Earth
Q21: A series LC circuit contains a 100
Q26: How many degrees of freedom is present
Q38: You can raise the temperature of an