The relation PV = nRT holds for all ideal gases. The additional relation PV holds for an adiabatic process. The figure below shows two curves: one is an adiabat and one is an isotherm. Each starts at the same pressure and volume. Which statement is correct? (Note: ' ' means 'is proportional to'.) 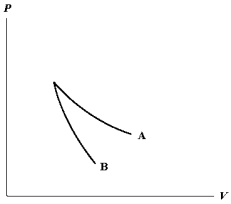
Definitions:
Minerals
Naturally occurring inorganic substances with a definite chemical composition and physical properties.
Real Estate
Property consisting of land or buildings, and anything affixed to the land, which can include residential, commercial, and agricultural properties.
Remove Soil
The act of excavating or relocating dirt, sand, or earth from one location to another, often for construction or landscaping purposes.
Trade Fixtures
Personal property that a tenant installs on a leased property for business purposes, which can be removed at the lease's end.
Q3: A wind of velocity 10 m/s
Q7: A body of mass 5.0 kg is
Q9: It is possible to hear an
Q14: Some species of whales can dive
Q14: A water pipe must deliver at least
Q20: Isaac Newton was able to estimate a
Q21: A 300-g glass thermometer initially at
Q29: A scuba diver has his lungs filled
Q73: _ can be defined as the study
Q108: Linguistic intelligence is most important for whom?<br>A)