The relation PV = nRT holds for all ideal gases. The additional relation PV holds for an adiabatic process. The figure below shows two curves: one is an adiabat and one is an isotherm. Each starts at the same pressure and volume. Which statement is correct? (Note: ' ' means 'is proportional to'.) 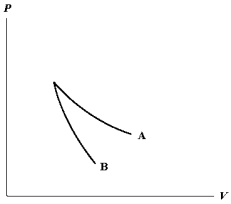
Definitions:
Millions
A numerical term used to denote a quantity of one thousand thousand (1,000,000).
Equilibrium Outcome
The state in which market supply and demand balance each other, and as a result, prices become stable.
Pure Strategies
A strategy in game theory in which a player makes a specific choice or takes a specific action with certainty.
Sponsor
An individual or organization that provides funds for a project or activity carried out by another, in exchange for advertising or promotion.
Q1: At the Australian War Memorial in Canberra,
Q3: Regardless of differences in religion, language, or
Q6: A Carnot cycle, operating as a heat
Q12: A steel bridge strut decreases in radius
Q12: An employee who just earned an MBA
Q13: A thin rod of mass M and
Q15: The duty of the board of directors
Q32: Suppose there are 3 molecules in a
Q66: the halo effect
Q114: The most significant source of change impacting