Definitions:
Strategic Planning
The process of defining an organization's direction and making decisions on allocating its resources to pursue this strategy, including its capital and people.
Vertical Communication
The process of information flow between different levels of an organization, both upwards from subordinates to superiors and downwards from superiors to subordinates.
Failures of Substance
Situations or outcomes where the actual performance or result significantly deviates from the intended or expected one, often negatively.
Poor Analysis
Inadequate or faulty examination and evaluation of data or information, leading to incorrect conclusions or understanding.
Q63: Mutual funds may contain<br>A) stocks only.<br>B) bonds
Q102: New classical economists say that a fully
Q103: Vilfredo is considering buying a house for
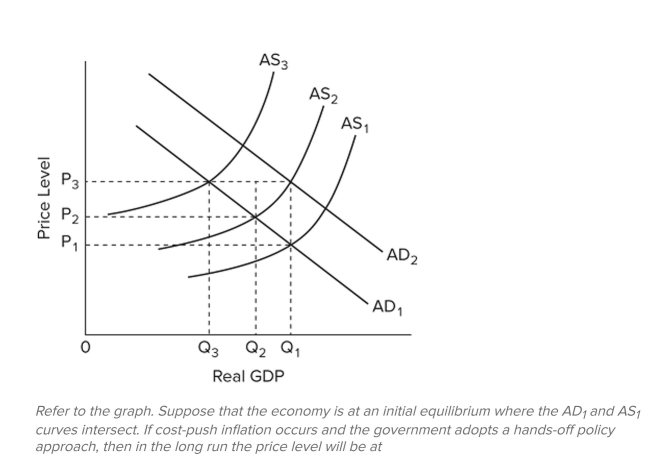
Q177: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8601/.jpg" alt=" Refer to the
Q205: In terms of aggregate supply, the short
Q226: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8601/.jpg" alt=" Refer
Q233: In 2012, the Fed adopted a flexible
Q238: The United States can be classified as
Q240: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8601/.jpg" alt=" Refer
Q250: Suppose stock A sells for $50 per