Multiply using a special product formula.
-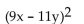
Definitions:
Partial Pressure
Partial pressure is the pressure that a gas in a mixture of gases would exert if it alone occupied the same volume at the same temperature, important in calculations involving gas laws.
Hemoglobin
A protein found in red blood cells that carries oxygen from the lungs to the rest of the body and returns carbon dioxide from the body to the lungs.
Dissociation Curve
A graphical representation of the relationship between the saturation of a substance (e.g., oxygen in hemoglobin) and the concentration or pressure of that substance.
Chloride Ions
Negatively charged ions (Cl-) that play critical roles in the body, including maintaining fluid balance and contributing to acid-base balance.
Q20: Jennifer Park's 2007 income was 6.9% greater
Q23: You inherit $70,000 from a very wealthy
Q62: 14 less than the product of 9
Q63: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A) f =
Q94: Use the formula <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Use the
Q102: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q118: (?, -4) for the equation 4x -
Q154: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A) (3x -
Q168: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A) (4x -
Q186: Consider two numbers. One of the numbers