Plot the point on the given axes.
-( 6 , 0) 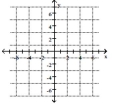
Definitions:
Atomic Orbitals
Formulas representing the whereabouts and wave-like characteristics of an electron in an atom.
Carbon-Carbon
Refers to bonds or interactions between carbon atoms within organic compounds; fundamental in organic chemistry and materials science.
σ Bonds
The strongest type of covalent chemical bond formed from the head-on overlapping of atomic orbitals, which allows for the free rotation of the bonded atoms around the bond axis.
σ Molecular Orbital
A type of molecular orbital formed by the head-on overlapping of atomic orbitals, characterized by electron density along the axis connecting the nuclei of the atoms involved.
Q5: (5, 1) and (8, 9)<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg"
Q6: -4x + 20y = 0<br>A) y =
Q13: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A) 1 B)
Q41: Over the years, State University has seen
Q47: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A) The first
Q118: (?, -4) for the equation 4x -
Q118: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q139: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q139: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q292: Suppose that a rectangular solid has length