Solve the problem.
-The pH of a solution ranges from 0 to 14. An acid has a pH less than 7. Pure water is neutral and has a pH of 7. The pH of a solution is given by 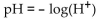 where
where 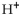 represents the concentration of the hydrogen ions in
represents the concentration of the hydrogen ions in
The solution in moles per liter. Find the pH if the hydrogen ion concentration is 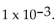
Definitions:
Competitive Advantage
The unique qualities or conditions that enable a business to outperform its competitors and achieve superior profitability.
Major Opportunities
Significant chances or openings that arise and can lead to growth, improvement, or competitive advantage if seized effectively.
Sustainable Competitive Advantage
is an enduring edge over competitors, achieved through unique resources or capabilities that are difficult for rivals to imitate.
Strategy Drives
The principle that strategic planning and decision-making guide the direction and actions of an organization.
Q31: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q31: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q36: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q61: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q93: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)
Q120: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A) 31 B)
Q126: 3p = 7(3p + 4)<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg"
Q153: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" Find the area.
Q199: f(x) = 4x, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="f(x) =
Q219: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt=" A)