Solve.
-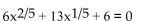
Definitions:
Zero-Order Reaction
A chemical reaction whose rate is independent of the concentration of the reactant(s).
Oxidation of Ethanol
The chemical reaction in which ethanol loses electrons or is combined with oxygen, typically forming acetic acid as a product.
Zero-Order Reaction
A chemical reaction whose rate does not depend on the concentration of the reactant(s).
Alcohol Dehydrogenase
An enzyme that facilitates the conversion of alcohols to aldehydes or ketones through the process of oxidation, playing a key role in metabolism.
Q23: Given <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8306/.jpg" alt="Given find
Q25: 10x + 2y = -4 5x -
Q38: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8306/.jpg" alt=" A)
Q45: {(-16, -4), (-14, -7), (18, 12)} <img
Q45: If galaxy A is four times more
Q47: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8306/.jpg" alt=" A)13y B)
Q64: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8306/.jpg" alt=" A)11 B)3, -3
Q77: A lot is in the shape of
Q79: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8306/.jpg" alt=" A)
Q113: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8306/.jpg" alt=" A)(5, 0), (-30,