Use the Table of Integrals to evaluate the integral. 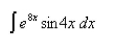
Definitions:
Activation Energy
The minimum amount of energy required to initiate a chemical reaction, determining the reaction rate.
Arrhenius Equation
A formula that gives the rate of a reaction as a function of temperature, providing a basis for understanding temperature effects on reaction rate constants.
Transition State
A high-energy configuration of atoms during a chemical reaction that represents the point of highest energy along the reaction path.
Energy Difference
The variation in energy between two states, configurations, or levels, important in understanding chemical reactions and physical processes.
Q25: The graph of the second derivative <img
Q40: Evaluate the integral using the indicated trigonometric
Q47: Find the area of the region under
Q61: Find the orthogonal trajectories of the family
Q78: Evaluate the integral.Select the correct Answer <img
Q85: Choose the differential equation corresponding to this
Q107: Find the number b such that the
Q111: Make a substitution to express the integrand
Q112: Evaluate the integral or show that it
Q158: Let <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8680/.jpg" alt="Let and