Evaluate.
-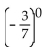
Definitions:
Reaction Rate
The speed at which a chemical reaction occurs, often influenced by factors such as temperature, concentration, and presence of catalysts.
Reaction Order
Indicates the relationship between the rate of a chemical reaction and the concentration of the reactants, used to predict reaction rates.
First-Order Reaction
A chemical reaction where the rate depends linearly on the concentration of one reactant.
Zero-Order Reaction
a chemical reaction in which the rate is independent of the concentration of the reactant(s).
Q51: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A) 8(x -
Q93: x + y = 14; (7, 4)<br>
Q98: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q103: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q123: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q146: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" " class="answers-bank-image
Q209: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q226: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A) 5 m
Q285: 630,000<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt="630,000 A)
Q286: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)