Multiply and simplify.
-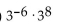
Definitions:
Proton Acceptor
A chemical entity that receives protons (hydrogen ions) in a chemical reaction, often measured in the context of acid-base chemistry.
Covalent Bond
The chemical bond involving shared pairs of electrons; may be single, double, or triple (with one, two, or three shared pairs of electrons, respectively). Compare with ionic bond and hydrogen bond.
OH− Concentration
A measure of the number of hydroxide ions in a solution, influencing its acidity or basicity.
pH
A scale used to specify the acidity or basicity of an aqueous solution, with values ranging from 0 (highly acidic) to 14 (highly basic) and 7 being neutral.
Q44: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q111: y = x - 3<br>A) (0, 3)<br>B)
Q120: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q152: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A) No common
Q167: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q178: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" In the
Q215: Wind resistance or atmospheric drag tends to
Q278: 8x + 3, when x = 5<br>A)
Q314: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q373: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)