Perform the indicated operation and, if possible, simplify.
-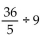
Definitions:
Orbitals
Equations detailing the wave-like properties of electrons surrounding an atom.
Orbitals
Mathematical equations that represent the wave-like properties of electrons within an atom.
Electrons
Subatomic particles with a negative electric charge, found in all atoms and acting as the primary carrier of electricity in solids.
Lewis Structure
A representation of molecules showing all the valence electrons and highlighting the bonding between atoms.
Q7: Reactive power <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8542/.jpg" alt="Reactive power
Q49: 1<br>A) 0.01%<br>B) 0.1%<br>C) 100%<br>D) 50%
Q53: 37.4%<br>A) 0.374<br>B) 0.00374<br>C) 374<br>D) 3.74
Q62: In solving series-parallel circuits using Ohmʹs law,
Q97: (14, 55) and (-65, -27)<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg"
Q110: x = 2.241<br>A) -3<br>B) -2<br>C) -2.241<br>D) 2.241
Q132: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A) -1 B)
Q160: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A) 3.4% B)
Q177: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)
Q210: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8504/.jpg" alt=" A)