The following questions refer to the diagram below.The levels represent energy levels in a hydrogen atom.Each level is labeled with its energy (above the ground state of Level 1)in units of electron/volts (eV).The labeled transitions represent an electron moving between energy levels.
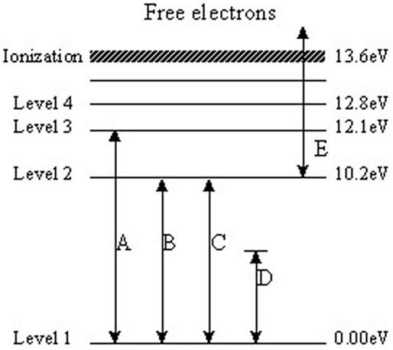
-Which transition represents the electron that emits a photon with the highest energy?
Definitions:
Aircraft Products Division
A specialized division within a company that focuses on the design, manufacture, and sale of aircraft and related products.
Antennae Division
The Antennae Division could denote a specialized segment within a company focused on the development, production, or distribution of antennae and related technologies; however, without context, its specific definition may vary.
Transfer Price
The price at which goods and services are sold between divisions within the same company or among affiliated companies.
Contribution Margin
The amount by which a product's sales revenue exceeds its variable costs, indicating how much contributes to covering fixed costs and generating profit.
Q7: From lowest energy to highest energy,which of
Q14: Which internal energy source is the most
Q33: He discovered that the orbits of planets
Q46: The Cassini mission to Saturn consists of<br>A)an
Q54: Suppose you discovered a meteorite that contains
Q61: Absolute zero is<br>A)0 Kelvin.<br>B)0° Celsius.<br>C)0° Fahrenheit.<br>D)100° Celsius.<br>
Q96: What does temperature measure?<br>A)the average mass of
Q106: Unbound orbits have more orbital energy than
Q107: Convection occurs in the troposphere but not
Q136: When an atom absorbs a photon containing