True/False
Molarity ( M )is calculated as: 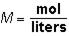 . M would be considered a derived unit.
. M would be considered a derived unit.
Definitions:
Related Questions
Q7: How many moles of K<sup>+</sup> and P<sup>3-</sup>
Q12: Which of the following represents a physical
Q23: Which of the following units of
Q45: Atoms or ions are considered isoelectronic if<br>A)they
Q57: Suppose the speedometer in your car reads
Q80: Oxalic acid is found in many plants,
Q82: Both bonding and non-bonding electron pairs can
Q89: The expansion of mercury in a thermometer
Q90: Calculate the kinetic energy of a 0.500
Q92: Explain the differences between a pure substance