Solve the equation. 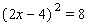
Definitions:
Reaction Rate
The speed at which a chemical reaction occurs, often influenced by factors such as temperature, concentration, and presence of catalysts.
Reaction Order
Indicates the relationship between the rate of a chemical reaction and the concentration of the reactants, used to predict reaction rates.
First-Order Reaction
A chemical reaction where the rate depends linearly on the concentration of one reactant.
Zero-Order Reaction
a chemical reaction in which the rate is independent of the concentration of the reactant(s).
Q4: A(n) _ dialog box typically displays the
Q18: The reference to an object that is
Q74: The difference of a number and 6
Q77: Simplify the complex fraction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8806/.jpg" alt="Simplify
Q126: Solve the equation by using the quadratic
Q135: Find the root. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8806/.jpg" alt="Find the
Q176: The number of seconds T it takes
Q196: Perform the indicated operation. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8806/.jpg" alt="Perform
Q217: Simplify the complex fraction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8806/.jpg" alt="Simplify
Q248: A bathtub can be filled by the