If the 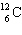 isotope had been assigned a mass of 6.0 u instead of 12 u, the weight of sulfur would be:
isotope had been assigned a mass of 6.0 u instead of 12 u, the weight of sulfur would be:
Definitions:
Hexane
A hydrocarbon with the chemical formula C6H14, used as a solvent in industrial applications, including the extraction of oils and fats.
Alkane Solvents
Organic solvents composed of alkanes, which are hydrocarbons containing only single bonds, making them non-polar and effective for dissolving similarly non-polar substances.
Electronegative
A measure of an atom's ability to attract and hold onto electrons when it forms bonds, affecting the polarity of chemical bonds.
Br
The chemical symbol for bromine, a halogen element with atomic number 35 that is a reddish-brown liquid at room temperature, used in various industrial applications.
Q10: When importing a Word document into Publisher
Q14: What is the balanced net ionic equation
Q17: How many moles of Na<sub>3</sub>PO<sub>4</sub> are present
Q34: What is the mass of 25.0 mL
Q42: Case James Lim asks his colleague, Georgia
Q50: Given the following data:<br>Fe<sub>2</sub>O<sub>3</sub> (s) + 3
Q65: Which neutral element from the list below
Q120: How many grams of sulfur , S,
Q139: The reaction P<sub>4</sub>+ 10 Cl<sub>2</sub>→4 PCl<sub>5</sub> is
Q160: What is the empirical formula of a