If 16.87 mL of 0.165 M NaOH solution is titrated witH49.70 mL of H₂ SO₄. What is the molar concentration of the H₂ SO₄solution?
2 NaOH (aq) + H₂ SO₄(aq) Na2 SO₄(aq) + 2 H₂ O ( 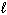 )
)
Definitions:
Psychological Tension
A mental or emotional strain resulting from conflicting, demanding, or challenging situations.
Creditworthiness
A valuation of the likelihood that a borrower can fulfill their financial obligations.
Economic Significance
Refers to the impact of a phenomenon, action, or policy on the economy's performance, often measured through its effects on growth, employment, and productivity.
Repeated Experience
An occurrence or event that happens multiple times, allowing individuals or organizations to gain insight or familiarity through its repetition.
Q13: How many mL of 1.0 M HCl
Q24: By observing the scattering of alpha particles
Q43: Consider the Acid-Base neutralization of sulfuric acid
Q47: What is the molarity of a NaOH
Q53: How many electrons (both bond and lone
Q55: An argon ion laser emits light at
Q77: Arrange the following bonds in order of
Q86: Choose the correct net ionic equation for
Q87: What is the partial pressure of Ar
Q98: What mass of CO<sub>2</sub> is produced by