Given the equation below, what is D H for the reaction that converts 6.00 g of H₂ O ( 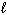 ) into H₂ O₂ (
) into H₂ O₂ ( 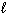 ) and H₂ (g) ?
) and H₂ (g) ?
2 H₂ O ( 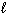 ) H₂ O₂ (
) H₂ O₂ ( 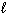 ) + H₂ (g) D H = 384 kJ
) + H₂ (g) D H = 384 kJ
Definitions:
Industry Demand Curve
A graphical representation showing the total demand for the products or services of a particular industry at various price levels.
Government Franchising
A process by which a government grants a company the exclusive right to operate, produce, or sell a service within a certain area.
Legal Barriers
Restrictions established by law or regulation that control how businesses operate, often to limit competition or protect consumers.
Short Run
In economics, a period in which at least one factor of production is fixed, allowing for limited adjustments to changes in demand or supply.
Q9: How many moles of H are present
Q28: Which atom listed below has the highest
Q36: What is the temperature given by the
Q42: Which bond listed below would be most
Q54: Arrange the following bonds in order of
Q71: A precise measurement<br>A) is known to two
Q73: What is the pressure in millimeters of
Q74: A chemical property of magnesium metal, Mg
Q75: A specially prepared sample of nitrogen contains
Q184: How many moles of sodium ions, Na<sup>+</sup>,