Given the equations shown below and their corresponding enthalpies:
H₂ O₂ ( 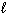 ) H₂ O (
) H₂ O ( 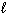 ) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O (
) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O ( 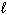 ) D H = - 285 kJ What is D H for the following reaction?
) D H = - 285 kJ What is D H for the following reaction?
H₂ O₂ ( 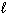 ) H₂ (g) + O₂(g)
) H₂ (g) + O₂(g)
Definitions:
After-Tax Borrowing Rate
The interest rate on borrowed funds after accounting for the effect of income taxes.
Cost of Capital
The rate of return that a company must earn on its investment projects to maintain its market value and attract funds.
Financial Lease
A lease agreement where the lessee effectively obtains all the risks and rewards of ownership without actually owning the asset.
Q20: The mass number of the only stable
Q34: What is the mass of 25.0 mL
Q46: What is the molar mass of potassium
Q51: What is the energy of a photon
Q52: Given the average bond energies listed below,
Q67: Which element has the valence electrons 4s<sup>2</sup>3d<sup>2</sup>?<br>A)
Q70: How many calories of heat energy are
Q74: Exhibit 8-1 The following question(s) relate to
Q102: What is the molarity of a NaOH
Q105: Which compound listed below is not soluble