Which of the following substances would be expected to have an enthalpy of formation value D H f equal to zero?
(Pay careful attention to the phase indicators.)
I. O₂(g)
II. N₂( 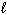 )
)
III. H₂ O ( 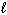 )
)
Definitions:
Invoice
A document issued by a seller to a buyer, listing goods or services provided, with their prices, and requesting payment.
EOM
EOM stands for "End of Month," which is a term often used in accounting and business to signify deadlines or the conclusion of financial periods.
Invoice
A document issued by a seller to a buyer that specifies the products or services provided and the corresponding payment terms.
Scratch-and-Save
A promotional discount method where customers scratch a card to reveal a discount or prize.
Q6: What is the pressure in a 1.00
Q24: Calculate the frequency of light whose wavelength
Q24: What is the bonded atom lone pair
Q43: What is the molarity of a solution
Q50: Which species below is largest?<br>A) Cl<br>B) S<sup>2
Q56: What mass of Al(OH)<sub>3</sub> is formed from
Q62: Which describes a set of elements on
Q67: What is the molar mass of a
Q85: What bonded atom lone pair arrangement is
Q126: The pressure of an ideal gas is