For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. N₂(g) + O₂(g) 2 NO (g)
II. C (s; graphite) + O₂(g) CO₂ (g)
III. H₂ O ( 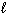 ) + CO₂ (g) H₂ CO3(
) + CO₂ (g) H₂ CO3( 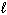 )
)
Definitions:
QuickBooks
An accounting software package developed and marketed by Intuit, designed for small and medium-sized businesses.
Compressed File
A file that has been reduced in size through compression algorithms, facilitating easier storage and transfer.
Data File
A digital file that stores data to be used by a computer application or system.
QuickBooks
Accounting software that provides tools for managing invoices, expenses, employees, and reports for small to medium businesses.
Q1: How many moles of argon gas are
Q7: What is the final temperature upon mixing
Q12: The ionic compound containing lithium and sulfate
Q14: What is the balanced net ionic equation
Q29: The total pressure exerted by a mixture
Q32: Consider the polyatomic ion, (SO<sub>3</sub>)<sup>2 - </sup>.
Q48: A compound containing only a metallic element
Q119: To account for the fact that the
Q132: A sample of CO gas has a
Q157: How much SO<sub>2</sub> is needed to react