What mass of ethane gas (C₂ H₆ ) must burn in excess oxygen to produce 2.00 10 3 kJ of heat, given the thermochemical equation:
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O ( 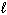 ) D H = - 3.12 10 3 kJ
) D H = - 3.12 10 3 kJ
Definitions:
Basic Product
The essential features or the core benefit that a consumer receives from a product, excluding any additional features, branding, or packaging.
Vertical Marketing Systems
Professionally managed and centrally coordinated marketing channels designed to achieve channel economies and maximum marketing impact.
Contractual
Relating to, secured by, or involving an agreement between two or more parties, especially one enforceable by law.
Corporate
Pertains to a large company or group, often structured legally as a corporation, and engages in various business activities.
Q6: Refer to Exhibit 5-1. How much heat
Q10: What mass of C<sub>4</sub>H<sub>10</sub> needs to be
Q18: The number 486,000 is equal to:<br>A) 4.86×10<sup>2</sup><br>B)
Q32: At constant temperature, a sample of 6.0
Q52: How many nanometers are present in 4.5
Q62: What is the pressure inside a 3.2
Q66: How many micrometers, m m, are present
Q86: The quantum number l for an electron
Q138: A fixed quantity of gas at 23.0
Q169: What is the sum of the coefficients