Given the thermochemical equations and their corresponding enthalpies:
CH4 (g) + 2 O₂(g) CO₂ (g) + 2 H₂ O ( 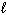 ) D H = - 890 kJ C₂ H4 (g) + 3 O₂(g) 2 CO₂ (g) + 2 H₂ O (
) D H = - 890 kJ C₂ H4 (g) + 3 O₂(g) 2 CO₂ (g) + 2 H₂ O ( 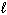 ) D H = - 1410 kJ What is the D H for the following reaction?
) D H = - 1410 kJ What is the D H for the following reaction?
2 CH4 (g) + O₂(g) C₂ H4 (g) + 2 H₂ O ( 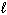 )
)
Definitions:
White Nose Syndrome
A fungal disease affecting hibernating bats, characterized by a white fungus on the nose and other body parts, leading to high mortality rates.
Pseudogymnoascus Destructans
A fungal pathogen known for causing White-nose syndrome, a deadly disease affecting hibernating bats.
Penicilium Chrysogenum
A species of fungus used in the production of the antibiotic penicillin.
Diverse Group
A category consisting of varying elements, individuals, or entities, characterized by differences in attributes such as appearance, behavior, or function.
Q14: The ground state electron configuration of Co
Q28: Direct reaction of P with Cl<sub>2</sub> can
Q32: At constant temperature, a sample of 6.0
Q33: Which element would be expected to have
Q46: Which of the elements from the list
Q52: What is the steric number with respect
Q69: Calculate the wavelength of light emitted when
Q74: Exhibit 8-1 The following question(s) relate to
Q76: Taking into account the size of ions
Q116: What is the steric number with respect