For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. N₂(g) + O₂(g) 2 NO (g)
II. C (s; graphite) + O₂(g) CO₂ (g)
III. H₂ O ( 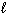 ) + CO₂ (g) H₂ CO3(
) + CO₂ (g) H₂ CO3( 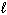 )
)
Definitions:
Mailbox Rule
A rule providing that an acceptance of an offer becomes effective on dispatch.
Air-conditioned Bus
A bus equipped with a system to cool and dehumidify the air, providing comfort to passengers.
Tour Group
A group of individuals organized for the purpose of traveling together, usually with a guide or planned itinerary.
Transact Business Electronically
The conduct of business activities through electronic means, such as the Internet or other digital platforms, rather than through physical exchanges or face-to-face.
Q4: Which of the following substances is expected
Q4: What is the volume in milliliters of
Q5: Which Lewis structure below obeys the octet
Q14: What is the correct ground state electronic
Q22: Which of the equations listed below corresponds
Q41: How many unpaired electrons does the ground
Q70: Arrange the ions Ca<sup>2+</sup>, Cl<sup>1 - </sup>,
Q75: A sample of carbon dioxide, CO<sub>2</sub>, gas
Q101: What mass of BaSO<sub>4</sub> precipitates from mixing
Q118: What is the ratio of A/B where