Multiple Choice
For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. 1 / 2 N₂(g) + 3 / 2 H₂ (g) NH₃(g)
II. H₂ (g) + Br₂( 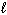 ) 2 HBr (g)
) 2 HBr (g)
III. HCl (g) + NH₃(g) NH ₄Cl (s)
Definitions:
Related Questions
Q6: Refer to Exhibit 5-1. How much heat
Q8: How many possible orientations are allowed when
Q17: What molecular shape is assumed by the
Q25: Three sets of quantum numbers are given
Q34: When 5.00 g of butane, C<sub>4</sub>H<sub>10</sub> (g),
Q40: Consider the reaction of sodium metal with
Q40: Using solubility guidelines, which of the following
Q76: The specific heat of water is 4.184
Q110: What is the empirical formula of a
Q185: Consider the following unbalanced reaction:<br>Al (s) +