Given the data below, what is D H for the following reaction?
C₂ H5 OH ( 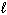 ) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O (
) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O ( 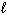 ) D H f C₂ H5 OH (
) D H f C₂ H5 OH ( 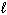 ) = - 278 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) = - 278 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 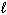 ) = - 286 kJ/mol
) = - 286 kJ/mol
Definitions:
Expensive Computer
High-cost computing device typically offering advanced capabilities and specifications.
Lease Assignment
The transfer of lease rights and obligations from the original tenant to another party.
Renewal Term
The period for which a contract or agreement, such as a lease or license, is extended beyond its original term.
Unpaid Rent
The amount of money that a tenant owes to a landlord for occupying property or space that has not been paid by the due date.
Q6: What is the pressure in a 1.00
Q6: What value listed below best approximates the
Q26: Which compound(s) below would be expected to
Q34: What is the mass in grams of
Q34: Listed below are three rankings of atoms
Q45: Given the following two equations and their
Q112: Which subatomic particles listed below are considered
Q119: What is the special name for the
Q125: How many moles of a gas occupy
Q139: What number of moles of hydrogen gas