A possible Lewis structure for BF 3 is given below. 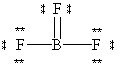 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
Definitions:
Defamation
False statements that injure a person’s reputation.
Corporate Performance
A measurement of a firm's efficiency and profitability in fulfilling its objectives and achieving its goals.
Serious Financial Trouble
A condition where a person, organization, or country faces significant financial challenges that threaten their long-term stability and solvency.
Excessive Response
Refers to a disproportionately intense or extreme reaction to a situation or stimulus.
Q9: At 20 ° C the Henry's law
Q13: The correct Lewis structure for Cl<sub>2</sub>CO is
Q41: Scientists of the 19th Century found that
Q48: Three series of atoms are listed below
Q66: What is the van't Hoff factor, "
Q75: Which of the following is true?<br>I. A
Q84: Pick the correct Lewis structure.<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q86: Consider the molecule shown below of carbonic
Q123: The concentration of O<sub>2</sub> in blood is
Q140: A gas is initially contained in a