A possible Lewis structure for BeCl₂ is given below. 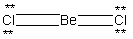 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
Definitions:
Ethnocentrism
Assessing a foreign culture purely through the lens of one's own cultural norms and values.
Cultural Competency
The ability to understand, communicate with, and effectively interact with people across cultures by acknowledging and respecting their beliefs and behaviors.
Culture
The shared beliefs, values, norms, and practices that characterize a group of people, shaping their behavior and understanding of the world.
Symbols
Visual, verbal, or written signs or objects that represent or stand for something else, often conveying ideas, concepts, or values.
Q22: What is the volume in liters of
Q26: What is the enthalpy of reaction D
Q55: Given the standard heats of formation listed
Q70: The Krypton atom in KrF<sub>2</sub> uses what
Q70: How many calories of heat energy are
Q72: Refer to Exhibit 13-2. What is the
Q79: What volume of H<sub>2</sub> gas at 27
Q99: The hybrid orbitals used by both N
Q149: What is the molality of a solution
Q150: What is the freezing point of a