A possible Lewis structure for BF 3 is given below. 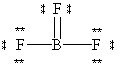 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
Definitions:
Birth Control
Methods, devices, or medications used to prevent pregnancy as a part of family planning, including contraceptives and sterilization procedures.
Legal Right
A defined entitlement protected by law that allows individuals to act in a certain way or requires others to act in relation to the holder.
Public Support
Refers to the backing or endorsement of policies, individuals, or causes by the general population.
Abortion
The termination of a pregnancy before the fetus can live independently, often invoked as a complex moral and legal issue.
Q6: What is the pressure in a 1.00
Q6: The element with a ground state electron
Q10: Given the three statements below, pick the
Q14: Gold crystallizes in a face-centered cubic array
Q15: Calculate the molarity of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) in
Q34: The hybrid orbitals used by Se in
Q42: What is the molarity of a HCl
Q53: Given the three statements below, which answer
Q83: The rate of a chemical reaction may
Q94: Which statement below best describes SO<sub>2 </sub>with