A possible Lewis structure for BeCl₂ is given below. 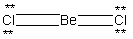 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
Definitions:
Consolability
The ability to soothe or comfort someone who is distressed, often used to assess emotional well-being.
Grimace
A facial expression often associated with pain, disgust, or disapproval.
Pain Scale Tools
Instruments or methods used to quantify or assess pain intensity, enabling better pain management and communication between patient and healthcare providers.
4-year-old Child
A child who has reached the age of four, typically experiencing significant development in areas like language, cognition, and social skills.
Q19: What is the transition from the gaseous
Q27: Which of the following phase changes is
Q57: Which series shown below is correctly arranged
Q63: Which of the following substances would be
Q67: The quantum number <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="The quantum
Q72: At 25 ° C, 13.0 mg of
Q74: What molecular shape would you predict for
Q76: The hybrid orbitals used by the nitrogen
Q119: What is the molarity of ions in
Q193: What is the freezing point for a