Below are two resonance forms of nitrogen monoxide. Which of the two structures is the preferred resonance form and why?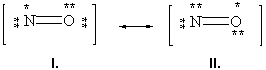
Definitions:
Net Capital Outflow
The difference between the domestic country's sale of assets to foreigners and the domestic purchases of foreign assets over a given period, usually indicating how much a country is investing abroad compared to foreign investments in the country.
Government Budget Deficit
A situation where a government spends more money than it receives in revenue over a particular period, often leading to the accumulation of debt.
Real Exchange Rate
The rate at which the goods and services of one country can be exchanged for the goods and services of another, adjusted for inflation.
Net Exports
The value of a country's total exports minus its total imports, representing the net effect of a country's international trade on its economic output.
Q7: What is the final temperature upon mixing
Q15: Which of the following reactions absorbs heat
Q20: Given the three statements below, pick the
Q21: The threshold frequency for the photoelectric effect
Q33: Three statements are made below. Pick the
Q40: What is the density of a sample
Q105: Pick the correct Lewis structure shown below.<br>A)
Q111: Predict the formula of an ionic compound
Q130: A chemical engineer places a mixture of
Q130: What molecular shape is assumed by the