Exhibit 13-12 Consider the aqueous reaction and data below to answer the following question(s) . 2 HgCl2 (aq) + C2O42 - (aq) →2 Cl - (aq) + 2 CO2 (g) + HgCl2 (s) 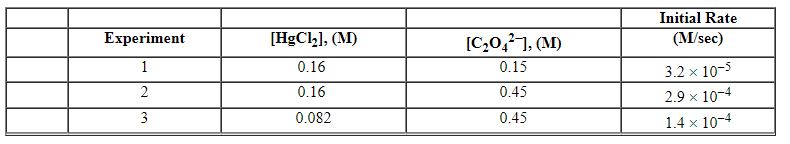
-Refer to Exhibit 13-12. What units would be used for the rate constant for the reaction above?
Definitions:
Rework Labor
The effort and cost associated with correcting defective goods or services after they have been produced.
External Failure Cost
Costs incurred when a product or service fails to meet quality standards after being delivered to the customer, including returns, repairs, and warranty claims.
Quality Cost Report
A document that details the costs associated with preventing, detecting, and correcting defective work in a company.
Warranty Period
The specific time frame during which a manufacturer or seller commits to repair or replace defective products.
Q19: What is the transition from the gaseous
Q28: At 25 ° C the vapor pressure
Q71: The gas phase reaction X + Z
Q72: Refer to Exhibit 13-2. What is the
Q79: A buffer solution maintains a constant pH
Q79: A solution of KOH is found to
Q81: The highly toxic pesticide parathion, (C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>PSOC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub>, decomposes
Q93: The gas-phase decomposition of SO<sub>2</sub>Cl<sub>2</sub> is a
Q100: What is the bonded atom lone pair
Q171: The molarity and molality of a solution:<br>A)