Consider the following first order reaction for the decomposition of dinitrogen pentoxide:
N₂O5(g) 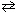 2 NO₂ (g) + O₂(g) At 70 C, the rate constant, k , for this reaction equals 6.82 * 10-3 s-1 . If the initial concentration of N₂O5 equals 0.0125 M, how long would it take for the concentration of N₂O5 to reduce to 0.00500 M?
2 NO₂ (g) + O₂(g) At 70 C, the rate constant, k , for this reaction equals 6.82 * 10-3 s-1 . If the initial concentration of N₂O5 equals 0.0125 M, how long would it take for the concentration of N₂O5 to reduce to 0.00500 M?
Definitions:
Soft Tissue Compression
The application of pressure to soft tissues, often used in medical treatments as a method to reduce swelling or improve circulation.
Tissue Layer
Different strata of cells that together form various organs and parts of the body, such as skin, muscle, and mucosal layers.
Adhere
To stick firmly to a surface or substance, or to follow closely or faithfully.
Nonblanchable Redness
A skin condition where red spots or patches do not turn white when pressed, often an early indicator of pressure injury.
Q53: What intermolecular force(s) of interaction is(are) possible
Q57: Consider a buffer solution containing a weak
Q72: What species are formed in the hydrolysis
Q74: Refer to Exhibit 13-12. What is the
Q90: What is the mole fraction of water
Q97: A sample of the radioactive isotope <sup>90</sup>Sr
Q130: For a reaction with the rate law
Q146: For the equilibrium reaction<br>AgCl (s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q183: The solubility of ethylene (C<sub>2</sub>H<sub>4</sub>) in water
Q199: One mole of HI is placed in