Given the following two reactions and their corresponding equilibrium constants:
N₂(g) + O₂(g) 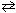 2 NO (g) K c1 = 4.3*10 -25 2 NO₂ (g)
2 NO (g) K c1 = 4.3*10 -25 2 NO₂ (g) 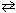 2 NO (g) + O₂(g) K c2 = 1.6 10 - 10 Combine these two reactions in a manner such that they sum to the net reaction that follows and then evaluate its equilibrium constant. N₂(g) + 2 O₂(g)
2 NO (g) + O₂(g) K c2 = 1.6 10 - 10 Combine these two reactions in a manner such that they sum to the net reaction that follows and then evaluate its equilibrium constant. N₂(g) + 2 O₂(g) 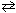 2 NO₂ (g) K net = ??
2 NO₂ (g) K net = ??
Definitions:
Double-Declining-Balance
A method of accelerated depreciation which doubles the normal depreciation rate.
Scrap Value
An estimated calculation of what an asset will be worth at the time it is sold, after it is no longer useful.
Sum-Of-The-Years-Digits
An accelerated method of depreciation which totals the digits of an asset's useful life and allocates the cost based on a fraction of those digits.
Scrap Value
The calculated resale value of an asset at the termination of its functional life.
Q1: When 20.0 grams of a water soluble
Q3: What is the pH of a solution
Q22: Carbonic acid is a diprotic acid with
Q66: Which of the following reactions is product
Q68: After carefully noting the orientation in which
Q77: Benzene boils at 80.10 ° C and
Q91: Refer to Exhibit 13-4. What is the
Q95: For most substances, the enthalpy of the
Q130: A solution of 3.0 g of citric
Q131: Refer to Exhibit 15-6. What is the