At 1000 K, the value of the K eq for the following reaction equals 0.338.
2 SO3(g) 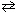 2 SO₂ (g) + O₂(g) K c = 0.338
2 SO₂ (g) + O₂(g) K c = 0.338
If the partial pressure values for these three components at a single moment were:
P SO3 = 0.16 atm P SO2 = 0.41 atm PO2 = 2.5 atm What can be stated about the reaction quotient , Q , and the direction that the reaction is preceding?
Definitions:
Q13: How many grams of water must be
Q30: What is the equilibrium constant expression for
Q31: Calculate the pOH of a solution made
Q47: The pH of a solution is 5.
Q76: Which of the following would have a
Q84: Which rate law given below corresponds to
Q88: Which of the following statements is incorrect
Q128: Select the best choice given the three
Q133: The following are factors that influence the
Q177: At 500 C, K p = 4.9*10<sup>-