When the reversible reaction, N₂+ O₂ 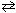 2 NO, has reached a state of equilibrium,
2 NO, has reached a state of equilibrium,
Definitions:
College Student
A person who is enrolled in an institution of higher education to pursue a degree or certificate.
Final Exam Week
A designated week at the end of a term or semester during which final exams are administered.
Mean Salary
The average amount of money earned by employees in a particular job, industry, or region, calculated by summing all salaries and dividing by the number of employees.
Median Salary
The middle value of salaries in a list when they are ranked in ascending or descending order.
Q14: A 3.60 m solution of KBr (molar
Q19: Iodine-123 is used to study thyroid gland
Q21: "A spontaneous change is always accompanied by
Q32: What is the conjugate acid of HBO<sub>3</sub><sup>2
Q35: Which of the items listed below would
Q40: If the reaction A + B→C has
Q90: What is the mole fraction of water
Q92: The reaction of carbon and water to
Q141: For NO + NO<sub>2</sub> + O<sub>2</sub>→NO<sub>3</sub> +
Q169: The equilibrium constant K c is 3.00